Convalescent plasma is in demand, but how does it help COVID-19 patients?

PHOTO: Amanda Solt
Testing on staging11
CHICAGO (NewsNation Now) — For the last 20 years, Amanda Solt has been on the front lines of health care in her hometown of LaGrange, Georgia. Solt, a paramedic nurse, treats patients at the immediate care clinic she runs with her husband.
She said her facility was 80% primary care treatment until the coronavirus pandemic hit.
For months, one patient after another walked into the clinic with COVID-19 symptoms. It quickly became a virus test center, she said.
“In June, a patient came in severely coughing, so I took him into the room to examine him,” Solt said. “I helped adjust the patient’s mask, I didn’t think anything of it since I was wearing full PPE, the gown, gloves and a mask.”
Within a week, Solt said COVID-19 symptoms began to set in, including coughing, shortness of breath, fatigue, body aches and loss of taste and smell. The 50 year old believes she contracted the virus from that sick patient because she wasn’t wearing eye protection.
“It got very hard to breathe. When I laid down on the bed, I felt like I was drowning,” Solt said. “At one point, I woke up with this feeling of impending doom, you know like something very bad, and I knew, it was time to go to the hospital.”

The mother of four was admitted into Piedmont Hospital, and she soon found herself in the intensive care unit (ICU). In and out of consciousness for days, Solt was awake at one point when her doctor asked her about convalescent plasma therapy.
Convalescent plasma therapy uses blood from people who’ve recovered from an illness to help others recover. In COVID-19 patients, the blood donated by people who’ve recovered from the virus has antibodies to the virus that caused the infection. The U.S. Food and Drug Administration (FDA) states convalescent plasma can be given to people with COVID-19 to boost their ability to fight the virus; however, it hasn’t been officially approved.
During this time of the pandemic, patients were being treated in different ways in hospitals around the country as part of an FDA program created to speed access to the experimental therapy.
“I was on oxygen and the nurse was holding up the phone to my ear so I can give a verbal consent to get convalescent plasma,” recalled Solt.
“I remember the day I got the plasma. My nurse took a selfie with the little bag of gold and said, ‘I want you to remember this moment that is going to save your life.'”
Amanda Solt, recipient of COVID-19 convalescent plasma treatment
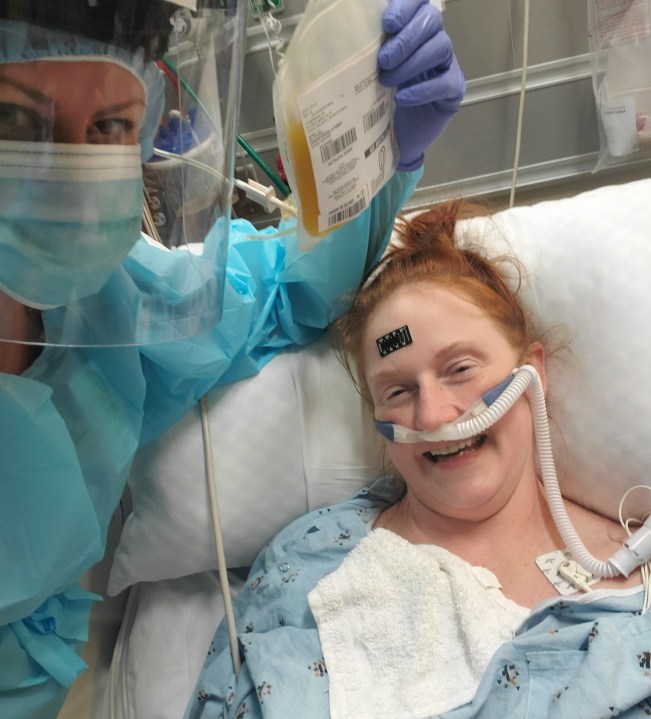
Solt said it was the convalescent plasma treatment that helped her stabilize and prevented her from being intubated.
“Literally, I got the plasma that my body needed to make the antibodies,” she said.
Within a week, Solt said she was out of the ICU and back in the hospital’s COVID-19 wing. After nearly four weeks of hospital care, Solt said she was able to go home, but she still experienced post-COVID-19 symptoms like hair loss, heart palpitations and loss of taste and smell.
“I feel so grateful to the person who donated the plasma. They were a risk-taker in trying to help,” Solt said. “I joke that I had Olympic gold medalist antibodies, I am going to pay it forward and donate my plasma, too.”
Convalescent plasma’s place in the COVID-19 fight
Success stories like Solt’s come as hospital distributions of convalescent plasma increased 250% in November compared to September, according to the American Red Cross.
The rise in hospitalizations of patients with coronavirus this fall and winter is the reason the Red Cross said it distributed a record number of COVID-19 plasma products to hospitals treating patients with the virus.
“We need donors across the board for two critical missions, to give blood and convalescent plasma,” said Paul Sullivan, senior vice president of the American Red Cross.
Sullivan said since the beginning of the pandemic, over 50,000 blood drives were canceled across the nation, impacting over 1 million blood donation appointments.
“We are sending out convalescent plasma at a greater rate and it’s a way for people to help those currently battling COVID-19,” said Sullivan.
In August, the FDA issued an emergency use authorization (EUA) for COVID-19 convalescent plasma for the treatment of hospitalized patients with the virus.
The same month, Mayo Clinic researchers reported that blood plasma from COVID-19 survivors helps other infected patients recover. But it wasn’t considered proof. In fact, there is no concrete evidence yet that convalescent plasma fights the coronavirus and, if so, how best to use it.
The Mayo Clinic reported preliminary data from 35,000 coronavirus patients treated with plasma, and said there were fewer deaths among people given plasma within three days of diagnosis, and also among those given plasma containing the highest levels of virus-fighting antibodies. However, it wasn’t a formal study.
Then in November, a study published in The New England Journal of Medicine found convalescent plasma did not significantly improve patients’ health status or reduce their risk of dying from COVID-19 severe pneumonia any better than a placebo.
After 30 days, researchers found no significant differences in patients’ symptoms or health. The mortality rate was nearly the same at 11% in the convalescent plasma group and 11.4% in the placebo group, a difference not considered statistically significant.
However, one of the study leaders said it is still possible that convalescent plasma might help less-sick COVID-19 patients, but more studies are needed to determine the effectiveness.
Doctor: Convalescent plasma is ‘not a miracle drug’
One convalescent plasma study currently underway is at Columbia University’s Irving Medical Center in New York.
Dr. Steven Spitalnik is leading a randomized controlled trial (RCT) on convalescent plasma with COVID-19 patients. He said what’s troubling about the wide use of convalescent plasma in this virus is the lack of trials that should’ve been conducted early on in the pandemic.
“Convalescent plasma is not a miracle drug like penicillin for pneumonia. If this has an effect, it is subtle,” said Dr. Spitalnik, who teaches pathology and cell biology at Columbia’s Vagelos College of Physicians and Surgeons. “We may have helped people or harmed them by making them worse but we won’t know because of how it’s been distributed without a traditional trial.”
Spitalnik said he was against the FDA’s guidance to use the therapy because it wasn’t monitoring results. He said there was a rush to temper the spread of coronavirus and to quell fears.
“I feel the push for convalescent plasma was politically driven and not based on the science to support it,” Spitalnik said. “People should be donating convalescent plasma and the message is to do the science correctly and then trust the science.”
The FDA lists common or possible side effects of COVID-19 convalescent plasma transfusion which include: allergic reactions, transfusion-associated circulatory overload, or lung damage with profound breathing difficulty, cardiac (heart) rhythm irregularities and blood clotting.
Spitalnik said it will take approximately three weeks from now to start analyzing the results from the convalescent plasma trial at Columbia University. He hopes it will reveal some concrete data about the effectiveness of the treatment.
For those interested in donating blood or convalescent plasma, go to The American Red Cross website, or call 1-800-Red-Cross to find a facility closest to your area.
If you would like to know more about convalescent plasma therapy, visit the FDA website.