(NewsNation) — President Joe Biden used Paxlovid to treat his mild symptoms when he tested positive for COVID-19.
The Food and Drug Administration granted emergency authorization for the pill, an antiviral therapy produced by Pfizer, in December. But while Paxlovid, intended to reduce the severity of COVID-19, was the first oral treatment approved for use against the virus in the U.S., it is not the only treatment available.
Here are some options people have for COVID-19 treatment, or prevention:
Paxlovid
Perhaps the most well-known drug available right now is Paxlovid. Available by prescription, state-licensed pharmacists can prescribe it to eligible patients. Paxlovid must be taken within five days after COVID-19 symptoms begin, per the FDA.
It is only authorized to treat patients 12 years old and up, who weigh at least 88 pounds after testing positive. It can be used in patients with mild to moderate cases, and who are at high risk of getting more severe COVID-19, including older people and those with health issues.
Paxlovid is given as three pills taken together twice a day for five days, two nirmatrelvir tablets and one ritonavir tablet. The Nirmatrelvir stops the virus from replicating, while ritonavir gives the first drug’s levels a boost by shutting down its metabolism in the liver.
According to Yale Medicine, Paxlovid can interact with many common medications, including over-the-counter treatments like St. John’s Wort, blood thinners, cholesterol medicines and more. Some common side effects can include altered or impaired taste, diarrhea, and increased blood or muscle aches.
Even with Paxlovid, though, there is a chance people could still test positive two to eight days after their initial recovery, and a return of symptoms is possible, according to John Hopkins Medicine. If this happens, the Centers for Disease Control and Prevention said, the protocol is for the patient to re-isolate for at least five days, until their fever has resolved for 24 hours without medication.
Molnupiravir
The National Insitute of Health recommends Molnupiravir, developed by Merck and Ridgeback Biotherapeutics, for non-hospitalized patients 18 years or older with mild to moderate COVID-19 who are at high risk of having the virus progress. The way it works is by blocking the ability of the COVID-19 virus to replicate in the bloodstream.

Yale Medicine says four capsules are taken every 12 hours for five days. The treatment should be taken as soon as possible, within five days of symptom onset. Side effects include diarrhea, nausea and dizziness. Molnupiravir is still being studied, so Yale notes that it’s possible that all of the risks aren’t yet known. Using it during pregnancy is not currently recommended, as it has not yet been studied in pregnant women and the risk of potential harm to a fetus is unknown. Also unknown is whether Molnupiravir could affect sperm.
NPR reported that Molnupiravir is a much less effective drug than Paxlovid, so doctors have been reluctant to prescribe it. (FDA data showed Molnupiravir was 30% effective at cutting the risk of hospitalization, while Paxlovid reduced it by nearly 90%.) Dr. Rajesh Gandhi, of Mass General Hospital in Boston, told NPR that he considers it an “alternative rather than a first-line drug.”
Remdesivir
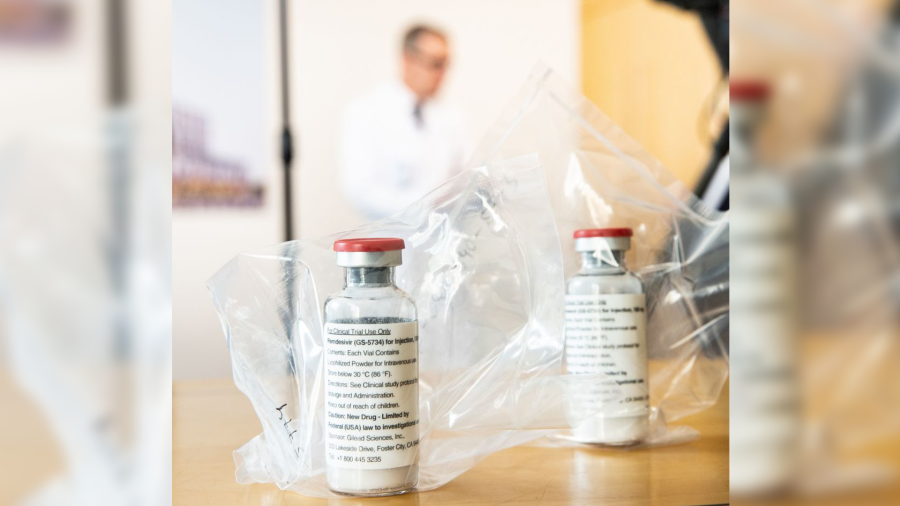
Remdesivir, also known as Veklury, was initially used in COVID-19 patients only after they were hospitalized, Yale Medicine notes, although new data has suggested it can be helpful in outpatients who become infected and are at high risk for severe disease. It is fully approved for high-risk children and adults. Children must be at least 28 days old, and weigh over 6.5 pounds or more.
Outpatients are treated over three days with a course of infusions that must be initiated within seven days of symptoms. Health care professionals are the only ones able to administer it, in a health care setting, Yale Medicine said.
The drug works by inserting itself into new viral genes to block replication of the coronavirus, thereby shortening how long it takes for ill patients to recover.
Nausea is the most common side effect, according to Yale, while hypersensitivity, including infusion-related and anaphylactic reactions, has been observed following treatment. Yale suggests pregnant or breastfeeding women consult their health care provider before taking it.
Bebtelovimab
Bebtelovimab is an investigational monoclonal antibody treatment developed by Eli Lilly, according to Yale. Although not all authorized antibodies have worked against COVID-19 variants, data shows Bebtelovimab’s efficacy against omicron and its BA.2 subvariant. Adults and children over 12 who weigh more than 88 pounds, have a positive COVID-19 test result and are at high risk for developing severe COVID-19 can get it. A prescription is required to get Bebtelovimab, according to the Department of Health and Human Services.
Given within seven days of symptoms starting, patients get an intravenous injection for at least 30 seconds. They are then observed by a health care provider for at least an hour after the injection.
Bebtelovimab binds to the spike protein that causes COVID-19 in order to fight it.
The FDA said there is limited information known about Bebtelovimab’s treatment of mild-to-moderate COVID-19. Allergic reactions the agency said to look out for include fever, difficulty breathing, chills, fatigue, fast or slow heart rate, chest discomfort or pain, weakness, confusion, nausea, headache, shortness of breath, facial swelling, itches or feeling faint.
Evusheld
The FDA says adults and adolescents over 12 years old who weigh at least 88 pounds and are at risk for severe illness can use Evusheld, a monoclonal antibody. Combining two drugs, Tixagevimab and Cilgavimab, it is not designed to treat COVID-19, but it does keep immunocompromised people who do not respond to vaccination from getting sick by combining two antibodies with different activities against COVID.
Health care providers give one dose of Evusheld in patients’ buttocks in two separate injections. Doses are repeated every six months that COVID-19 remains in circulation. Injection can cause hypersensitivity, pain, bruising, soreness, swelling, possible bleeding, or infection at the site where the dose was administered. Clinical trials saw serious but uncommon cardiac adverse events, Yale said.
Of course, most health officials, including the Centers for Disease Control, note that the best way to prevent hospitalization and serious illness from the virus is to get vaccinated.

